In this article, We will talk about science words that start with the letter ‘C’ and some of its scientific meanings.
Glossary of Science Words that start with ‘C’
As we are learning science words alphabetically, today let us explore the words that start with the letter ‘C’.
This post contains:
Chemistry words that start with ‘C’
Scientific generic terms and instruments starting with C

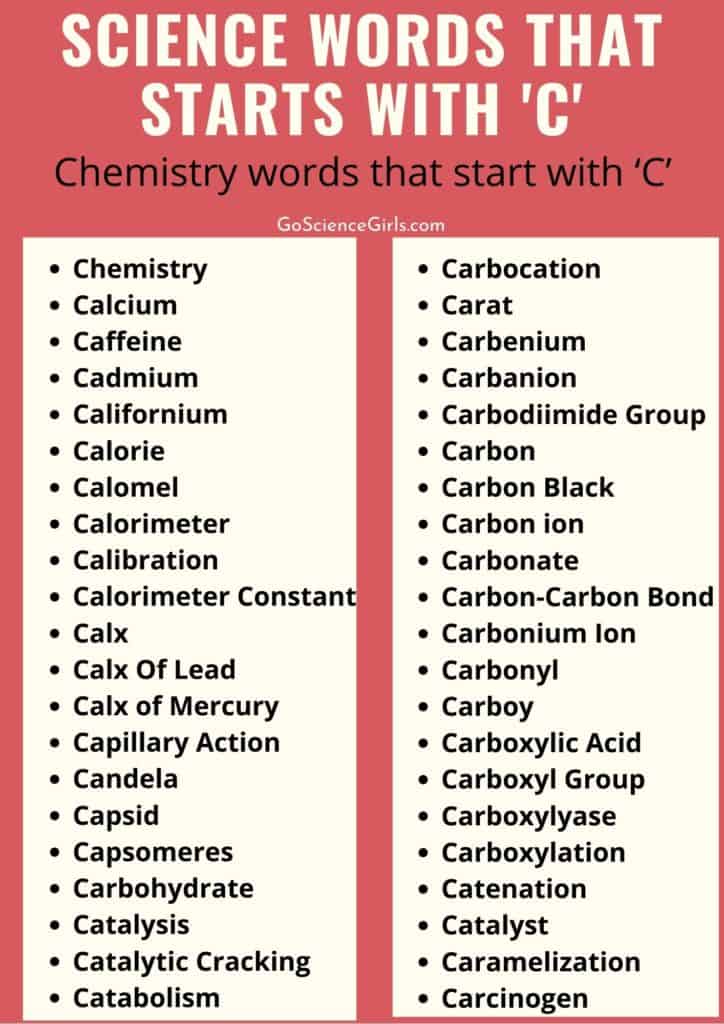
Chemistry words that start with ‘C’
Chemistry: Chemistry is one of the science branches that deal with compounds and elements, encompassing electrons, atoms, ions, and molecules. And investigates these chemical compounds in regards to their properties and reactions on their way to forming new substances. Biologically, it is a psychological relation and interaction between living animals including humans.
Calcium: Calcium is one of the earth’s elements that are basic in nature. And it has 20 atomic numbers in the periodic table holding the symbol ‘Ca’.
On the other hand, calcium is a nutrient mineral consisting of salts that are helpful in treating a lot many medical conditions. Because calcium is one such mineral required by all living organisms together with humans. Well in humans, calcium plays a hero role as it occupies a major portion of the body and maintains bone health. In addition to bone health, calcium contributes to clotting our blood, contracting muscles, and controlling the heartbeat.
Caffeine: Caffeine is a chemical but natural substance that stimulates the central nervous system and restores alertness psychologically. We can find caffeine in coffee, tea, and plant seeds are the main source of caffeine. In addition, we can find caffeine in many medicinal drugs that treat migraine and headache issues. As well as in dietary foods caffeine is present in little amounts, especially in dietary food and other such energy drinks.
We can find calcium in our daily intake of food in different forms such as green leafy vegetables, milk, cheese, and other dairy products.
Cadmium: Cadmium is a white, soft, and silver chemical element that occupies 48th place on the periodic table and holds the symbol ‘Cd’. Chemically, cadmium matches its properties to two other chemical elements in the periodic table i.e. zinc and mercury. Cadmium always exists in the natural environment in combination with other metals,s especially zinc and hence it is not a pure element. As it is toxic in nature, humans cannot use it for their bodies.
Californium: It is a chemical element but radioactive with atomic number 98 and symbol ‘Cf’. As it is a strong neutron emitter, it is useful in making portable metal detectors as well as detecting water and oil layers in oil wells, gold and silver ores, and identifying stress and metal fatigue in airplanes.
Calorie: The unit of energy is ‘Calorie’. In measurements, a calorie is the amount of energy useful for raising the temperature of a quantity of water by one degree.
If humans talk about calories in food, it means the measurement of the amount of energy gained or lost through the intake of solid and liquid food. Metabolism is the biological process through which the consumed food converts into energy. In this process, calories in food make use of oxygen in the body in order to release energy which is very much useful for body functioning.
Calomel: Calomel, otherwise known as mercurous chloride is a mineral holding the chemical formula, Hg₂Cl₂. Basically, it is a secondary and substitute product while depositing mercury. However, it is very much useful in treating malaria and yellow fever. Also, calomel used in making worm candy or wormchocolate helps in treating patients suffering with helminths.
Calorimeter: Calorimetry is the procedure of measuring the amount of heat flow or heat capacity involved during chemical reactions and physical changes. Well, a calorimeter is a measuring apparatus in the calorimetry process and gives accurate results. There are many kinds of calorimeters depending on the type of heat measured. But the most common types of calorimeters are titration calorimeters, Differential scanning calorimeters, accelerated rate calorimeters, and isothermal micro calorimeters.
Calibration: Calibration is the most significant method useful for determining measurement values of a device under test (DUT) using a standard reference value. To put it simply, this process helps in determining the input and output of a device depending on its function and reaction to the instructions given.
Calorimeter Constant (C): Calorimeter constant holds the symbol ‘C’ and calculates the calorimeter heat capacity. Basically, the calorimeter constant is derived when a constant amount of heat is applied to a calorimeter. That is when the change in reaction of the device is captured and measured calorimeter constant with the corresponding changes in temperature.
Calx: Calx, is a powdery metallic oxide formed from heated ore or mineral. The Calx of a metal is an oxide material. According to early chemical history, calx is a true elemental substance that lost its phlogiston during its combustion process.
Calx of Lead: Calx of Lead, is a deprecated chemical term for lead oxide compounds.
Calx of Mercury: Calx of Mercury, deprecated term for mercury oxide, HgO compound. Red calx of mercury is the other name for it.
Capillary Action: The ability of a liquid to move through a narrow and porous material without the help of any external forces like gravity is capillary action. This capacity of liquid movement through narrow spaces is due to the internal forces between the liquid and the tube material. Well, those internal forces include surface tension and cohesive and adhesive forces. Mostly, capillary action happens when the adhesive force of the intact material is more than the liquid cohesive forces.
Candela: The basic SI unit of luminous intensity in the International System is Candela. The symbol of the candela is ‘Cd’. The radius intensity of a one-track chromatic source that emits 540 × 1012 Hz light has a radiant intensity of 1/683 watts per steradian.
Capsid: The capsid is the outer protein layer that covers the genetic material of a virus. It mainly performs three functions: 1) caring for the nucleic acid from digestive enzymes 2) helping in attaching the virions to the host cell through its surface special sites3) making proteins available that help the virion to enter the host cell membrane.
Capsomeres: The subunit of the capsid is the capsomere, which also helps in covering the genetic material of the virus. We can see capsomeres mostly on the outer layer of the protein shell of the virus. Capsomeres consist of protomers that make up their content of it. And these capsomeres assemble themselves to build up capsid. Helical, Icosahedral, and complex are the different types of capsomere arrangements.
Carbohydrate: Carbohydrates are a large group of biomolecules or organic compounds consisting of oxygen and hydrogen molecules. These hydrogen and oxygen molecules bond together in the ratio of 2:1 same as the water molecule hydrogen-oxygen bond. And further, these bonds broke down and release energy to the living bodies including humans. We can see carbohydrates in certain foods and living tissues. Finally, the chemical formula of carbohydrates is Cm(H2O)n. Examples of carbohydrates include starch, glucose, fructose, cellulose, etc.
Carbocation: The positively charged carbon atom is a carbocation and it contains three bonds. Well, the alternate and well-known name for carbocation is carbon cation. However, it was earlier famous with the name carbonium ion. Examples of carbocations are methanium CH⁺ ₅, methenium CH⁺ ₃, and vinyl C ₂H⁺ ₃ cations.
Carat: Carat, is a measuring unit of gemstones, pearls, and gold. This measuring unit is generally equal to 200 milligrams or 0.00643 troy oz. the other name for carat is the metric carat. And the symbol of the carat is ‘Ct’.
Carbenium: Carbenium ions are the ions and highly reactive in nature because they need to be stabilized as it consists of three valence electrons i.e. R3C+. These ions can act together with polymers, monomers, solvent molecules, and anions.
Carbanion: A carbanion, is an anion in which the carbon atom holds three bonds and a negative charge. Officially, it is a conjugate base of a carbon acid with the chemical formula: R₃CH +:B⁻ → R₃C:⁻ + HB; here B denotes the base.
Carbodiimide Group: In organic chemistry, the universal IUPAC name of the carbodiimide group is ‘Methanediimine’. It holds the chemical formula RN=C=NR and it is a condensing reagent and synthetic functional group. The best example of a carbodiimide group is ‘dicyclohexylcarbodiimide’. This one is very much useful in making peptides.
Carbon: Carbon is a plentiful chemical element that mostly occurs in pure forms such as graphite, diamonds, etc. It has atomic number 6 in the periodic table with the symbol ‘C’. By nature, a carbon atom is tetravalent and hence it forms covalent bonds offering four electrons. And 0.025% of Soil’s crust is of carbon atoms. In addition, carbon in carbon dioxide plays a major role in the greenhouse effect.
Carbon Black: The residual material formed due to the incomplete combustion of heavy petroleum products is carbon black. These petroleum products include coal, tar, FCC tar, etc. It contains high coloring properties and is hence used in printing press papers, in rubber production as a reinforcing agent, and other such things. However, carbon black is not safe to inhale or expose to because it causes lung and even can irritate your eyes, throat, and nose.
Carbonate: In chemistry, carbonate is the salt content of carbonic acid. The chemical formula of carbonate is CO²⁻ ₃ and it contains a polyatomic ion and a carbonate ion. Well, it is a combination of one carbon atom and three oxygen atoms. Any compound that has a carbonate ion is generally named carbonate.
Carbonate Ion: The polyatomic ion with the chemical formula CO3(2-) is a carbonate ion.
Carbon-Carbon Bond: The bond existing between two carbon atoms is a carbon-carbon bond. In general, all the C-C bonds are covalent bonds. There are three types of carbon-carbon bonds such as carbon-carbon single, double and triple bonds. The single bond of two carbon atoms is generally a sigma bond which consists of two electrons. While the natural fatty acids reveal carbon-carbon double bonds. Half of the total fatty acids in the human body consist of C-C double bonds. The bond formed between an sp2-hybridized orbital and a p-orbital (which is not involved in the hybridization) is a C-C double bond. These bonds consist of unsaturated hydrocarbons with the chemical formula CnH2n. On the other hand, the bond formed between the sp-hybridized orbital and two p-orbitals from each atom is a C-C triple bond.
Carbonium Ion: The carbocations which hold five to six valence electrons are carbonium ions.
Carbonyl: Carbonyl is a divalent functional group with the molecular formula ‘RCOR’. This group consists of one carbon atom interacting with an oxygen atom in double bonds i.e. C=O. The molecules that belong to this group have prefix names such as keto- or oxo-. The other names for this group of compounds are the carbonyl group and the carbonyl functional group. Also, the compounds that consist of a metal bonded with carbon monoxide are considered carbonyl.
Carboy: Carboy, otherwise known as demijohn is a large spherical bottle of glass material with a narrow neck shape. It is generally useful for holding different types of liquids such as acids, water, corrosives, etc. And also widely used in fermenting beverages like beer, wine, etc., especially when made at home.
Carboxylic Acid: A carboxylic acid is an acidic group of carboxyls attaching to an R group. And thus the general formula of it is R-COOH. Here in this formula, R stands for aryl, alkenyl, alkyl, or other such compounds. Amino acids and fatty acids are the best examples of carboxylic groups of acids.
Carboxyl Group: The carbon-based functional group in which carbon atom bonded with oxygen atom using a double bond and hydroxyl group using a single bond. The molecular formula of carboxyl group compounds is -C(=O)OH or –COOH.
Carboxylase: Carboxy-lyases or decarboxylases are the lyases that contain carbon-carbon bonds to catalyze organic chemical reactions. Carboxylyases only catalyze the reactions that involve organic compounds from which carboxyl groups add or remove.
Carboxylation: The chemical reaction between a substrate and carbon dioxide in which the residual product is a carboxylic acid group.
Catenation: Catenation is a chemical linkage of elements that contain a similar number of atoms. These linkages of chains form only between atoms of an element that contains at least two valence electrons. Well, such kinds of linkages generally form strong bonds and carbon is the best element to exhibit the catenation process. And it is popular for making elongated hydrocarbon chains plus rings like benzene.
Catalyst: Catalyst is a chemical substance that exhibits chemical properties and acts as a barrier in a chemical reaction in order to increase its rate of reaction. But it never undergoes any changes in between the chemical reaction either temporarily or permanently. For example: to break down ozone, chlorine acts as a catalyst. Catalysts are in demand for very little quantities in order to catalyze any chemical reaction.
Catalysis: The method of increasing the rate of a chemical reaction by adding a little amounts of catalyst is a catalysis process. And it is an effective and quick process of converting resources into essential products utilizing very less amounts of energy and providing less wastage. Homogenous, heterogeneous, Heterogenized homogeneous, and biocatalysts are the different types of catalysts and catalysis processes.
Catalytic Cracking: Catalytic cracking is the most significant method of cracking reactions of products especially petroleum refineries in the presence of a catalyst. In the oil industry, this method is useful in cracking heavier compounds into lighter and movable products with the help of catalysts.
Catabolism: The process of breaking down of complex materials in living organisms using a set of metabolic pathways into smaller units is a catabolism process. And this conversion process releases energy by oxidizing and is even used in other anabolic reactions. Mostly, these reactions use enzymes as a catalyst in order to increase the reaction.
Caramelization: Caramelizationis the method of breaking down sugars either in the crystallized form or in the food using high temperatures. Such that the sugars break down into fructose and glucose by removing water content. During this process, the sugars take brown color and are hence used in various cooking methods in order to add that brown and sweet flavor. And the brown color is the result of the action of three polymers such as caramelens, caramelans, and caramelins.
Carcinogen: Carcinogens are the intermediate agents that have the capacity to cause carcinogenesis in humans. Carcinogenesis is the process of developing cancer cells in the body which results in causing cancer. Carcinogens work in carcinogenesis by acting on the DNA of the cell that makes mutations in genetic material. And these cancer-causing agents are natural for example, aflatoxin. We can find Aflatoxin on the preserved grain surfaces, and even fungus can produce them. Also, carcinogens are man-made for example, tobacco smoke and asbestos.
Carotenoid: Carotenoids are the fat-soluble organic pigments available in orange, yellow, and red colors in order to give color to the plant parts. For example, autumn leaves and colored fruits or flowers gain pigmentation through carotenoids. Not only plants, but different forms of algae, fungi, and bacteria can also give pigmentation through carotenoids. For Example Tomatoes, Flamingos, carrots, pumpkin, salmon, etc.
Cathode: The cathode is one of the electrodes of an electronic device through which electrons enter inside it by leaving the conventional current. Basically, the electric charges on the cathode differ and act according to the movement of conventional energy. And during release, the positive electrode is the cathode and the negative electrode is the anode.
Cation: Cation is the ion that holds a positive charge and attracts to the cathode during the electrolysis process. Coming to the protons and electrons, a cation consists of more protons than the number of electrons. And that’s the reason, cation holds a positive charge. Atoms that tend to gain stability and have strong attraction typically drag the electrons and result in the formation of cations.
Cathode Ray Tube: Cathode-Ray tube, is a high-vacuum tube that uses manipulated beams of rays in order to produce luminous images through electronic guns on phosphorescent or fluorescent screens. You can see these tubes in our homes appliances like televisions and screens of computers. And the images are seen in the form of radar targets, waveforms, and pictures.
Cathode Ray: Cathode rays, otherwise known as e-beam or electron beams because it is a beam of emitting electrons into the vacuum tubes. These beams of electrons result in the formation of a negative electrode in the vacuum tube where there is low-pressure gas.
Causality Principle: This is the important principle of science that establishes the relationship between the causes and their effects. It states the logical reaction of a cause between any two incidents and the preceding effect.
Cell Potential: The possible difference occurring in an electrochemical cell between the two half cells i.e. anode and cathode is the cell potential.
Cavitation: The method of collapsing air bubbles in a liquid by boiling and reducing the pressure is cavitation. Such that there is the formation of small cavities in a liquid in the areas where there is less pressure.
Celsius Temperature Scale: Celsius temperature scale, was invented in 1742 by Andres Celsius who was an astronomer from Sweden. Basically, it is a scale representing the water’s freezing point of 0 degrees and boiling point of 100 degrees. This scale is alternatively known as the centigrade or Celsius scale as there is a 100-degree difference between the distinct points. And these freezing and boiling points are standard ones a standard atmosphere.
Centi: Centi is the prefix name but actually represents the unit of measurement that represents the one hundredth. The symbol of centi is ‘C’ and is related to x10-2.
The breadth of a small eraser is around 1 centimeter or 1⁄100 m
Ceiling Limit: Ceiling limits are the substance’s concentration levels or values to which a person should not get exposed for long hours. Because when a person is exposed to beyond these limit values, he/she might fall ill with several medical issues which sometimes results in death.
For example: Exposing to ammonia for more than 5 minutes may cause ill health because the ceiling limit of ammonia is 50 parts per million.
Ceramic: The outcome of mixing non-metallic material i.e. clay with powders, water, and other earthen elements and then heating it is ceramic. One need to heat these materials in order to make ceramic hard, heat, and corrosion-resistant at high temperature. Well, the three types of ceramic are stoneware, earthenware, and porcelain. In the market, ceramics are amazing that are available in different designs and shapes and works best as home or office decorative.
Cesium: Cesium is a soft and gold color metal yet dangerous chemical element with atomic number 55 and the symbol ‘Cs’. Basically, it is alkaline in nature and useful in making extraordinary optical glasses, as a catalyst and drilling fluid, and also in radiation checking devices.
CFC: A particular class of compounds that only contain chlorine, fluorine, hydrogen, and carbon elements is CFCs. These compounds are famous for the brand name ‘Freon’ and are commonly useful in aerosol propellants and refrigerants. Besides, these compounds are not good for Earth’s protection layer, Ozone.
Cerium: Cerium is one of the most abundant rare earth chemical elements holding the atomic number 58 and the symbol is Ce. This lanthanide element is useful in making cigarette lighter alloys. And its oxides are useful in making glass polishing agents and as catalysts in cleaning ovens.
Cetane Number (CN): Cetane Number is the limit value that indicates the combustion speed of fuels, especially diesel, and also measures the compression useful for combustion. It plays important role in determining the diesel fuel quality.
CGS Units: CGS in full form says, Centimetre-Gram-Second. As the name itself suggests, it is the unit measurement commonly useful for measuring things in the metric system. And these measurements depend on a centimeter-scale where ‘G’ represents the unit of mass, the centimeter represents the length, and the second represents the time unit.
Chain Reaction: Chain reactions are mostly visible in physical and chemical sciences. The sequential reactions whose products continue to be the reactants of other series of reactions are chain reactions. These reactions generally happen in the absence of external factors.
Chain Molecule: Any class of molecules made up of a sequential order of atoms or elements which binds together strongly are chain molecules. All the simple hydrocarbons are chain molecules.
Chalcogen: Chalcogens are oxygen family because it consists of chemical elements belonging to group 16 of the periodic table. These elements encompassed selenium, oxygen, tellurium, sulphur, and polonium, a radioactive element.
Chalcogenide: The chemical compounds that contain at least one chalcogen anion along with at least one electropositive element are chalcogenides.
Charles’s Law: Charle’s Law, is also known as the law of volumes because it states the volume occupied by a fixed amount of gas at constant pressure. It is an ideal experimental gas with a formula that proves volume is directly proportional to temperature. In the formula, I represent the initial.
Vi/Ti = Vf/Tf
Charge: The electric charges that represents the electromagnetic interactions between the subatomic particles are generally referred to as a ‘Charge’. For example: electrons have –I charge whereas protons have +1 charge by convention.
Chaotropic: The compound which helps in disturbing the hydrogen bonds between water molecules in the aqueous solutions is referred to as chaotropic agents. Basically, these agents weaken the hydrophobic influence, which is very important in making 3D structures of macromolecules like nucleic acids and proteins.
Chelate: The process of attachment of many molecules and ions to ions of the metals is chelation. The organic compound formed during the chelation of ligand bonding to a metal ion is a chelate.
Chelating Agent: The ligand which has the capacity to form a chelate through the linkage of metal atoms is a chelating agent.
Chemical Energy: The energy required by a chemical element to convert into another substance through chemical reactions is chemical energy. Also, the energy released during the internal changes of an atom or molecule is chemical energy.
Chemical Equation: This is an equation that represents the reactions happening in a chemical reaction using symbols and molecular formulas of respective reactants.
Chemical Reaction: The reaction happening between two or more molecules or atoms chemically is a chemical reaction. These reactions result in the formation of new products and by-products.
Chemical Change: The changes happening between two or more reactants in a chemical reaction in order to form new or different products are chemical changes.
Chemical: Chemical is a word related to the science of chemistry which must contain distinct chemical properties and stable composition. To keep it simple, the chemical is a compound that has mass. For Example Any solids, liquids, or gases like water, pencil, bubbles, etc.
Chemical Engineering: It is a branch of applied chemistry that applies the principles of chemistry to raw materials and converts them into useful products such as paper, clothes, food, drinks, etc. Those who design and develop chemical manufacturing methods for the improvement of newly formed products are chemical engineers.
Chemical Equilibrium: It is a state of condition in a chemical equation where reactants or products don’t tend to react with other molecules because already there exists stability in their concentrations. If you say any reaction attained chemical equilibrium means there is no noticeable change in the properties of the reactants.
Chemical Property: The performance or property of a reactant while undergoing a chemical reaction or change is a chemical property. These properties may refer to toxicity, oxidation or reduction capacity or tendency to catch flame, etc.
Chemical Physics: This is the branch of physical chemistry where studies happen using chemical principles along with molecular physics. Thermodynamics, quantum chemistry, quantum mechanics, statistical kinetics, and mechanics are a few of the principles helpful in studying chemical processes.
Chemical Kinetics: Chemical kinetics is a branch of physical chemistry, also popular with other names i.e. Reaction Kinetics. This branch studies the rate of reactions that are happening chemically such that one can understand whether the reaction is going fast or slow.
Chemical Formula: It is a formula which tells about the reactants chemical composition in regards to elements symbols, and their atomic numbers along with other symbols like +, -, =, brackets, etc.
Chemical Symbol: The alphabetical short forms or abbreviations used to represent chemical compounds, elements, and functional groups in chemistry are chemical symbols. The alternate popular name for these symbols is element symbols and usually consists of one or two letters.
Ex: The chemical symbol of carbon is ‘C’, hydrogen is ‘H’, etc.
Chiral Centre: Basically, it is an atom in a molecule that tends to bond with other chemical atoms on all four sides using different ligands and attains optical isomerism. If the tetrahedral atom is carbon forming center of chirality, it is otherwise known as asymmetric carbon.
Chirality: Chirality is an important asymmetry property useful in many branches of science. In mathematics, it is a property where a figure is un-identical to its mirror image. Besides, it is a property of a pair of pf molecules that share the same formulas but differences in their structures in chemistry.
Cherenkov Radiation: Cherenkov radiation, also known as Cerenkov radiation is an electromagnetic emission of light from electrically charged atoms or elements. These radiations emit from charged particles when passed through dielectric medium with the higher speed than the light velocity.
Chemiluminescence: The light emitted as a result of a chemical reaction is the chemiluminescence process. It is mainly and highly useful for solving crime issues in the forensic department.
Chlorate: The salt or ester forms of chloric acid compounds are chlorate anions. It has the chemical formula, ClO⁻ ₃. Also, the chemical compounds that contain these formulated structures are chlorates.
Chlorinated Hydrocarbon: The compounds that contain carbon, chlorine, and hydrogen atoms are chlorinated hydrocarbons. To put it simply, a hydrocarbon molecule in which one or more hydrogen atoms are substituted with chlorine atoms can refer as a chlorinated hydrocarbon.
Chloride: Any chemical compound in which chlorine atoms bonded covalently within the molecule are chlorides. Also, the chlorine atoms in the salts of hydrochloric acid, HCl termed chlorides.
Chlorination: This is a chemical disinfection process useful for purifying water by killing viruses, bacteria, and other microbes. Basically, it is helpful in avoiding the spread of waterborne infections by oxidizing organic contaminants. However, chemically it is a process of combining at least one chlorine atom into a substance. And this process of integration of chlorine atoms into a substance is a chlorination reaction.
Chlorocarbon: Chlorocarbons are nothing but hydrocarbons that tend to substitute their hydrogen atoms with chlorine atoms. There are seven chlorocarbons in chemistry among which four are chlorinated C1 hydrocarbons that fall under the methane family and the other three are chlorinated C2 hydrocarbons that fall under the ethane family.
Chlorite: The chemical substances that comprise at least one chlorite anion are chlorites. These anions take the molecular formula ClO2–.
Chlorine: Chlorine is one of the naturally occurring lightest halogen elements with atomic number 17 and the symbol ‘Cl’ in the periodic table. It takes second place in regards to its lightness among all halogens and occupies the place between fluorine and bromine in the table. And it shows the properties of both the neighboring elements and appears as a yellow-green gas from outside at room temperature.
Chromosphere: The atmosphere around the sun is generally divided into three layers and the chromosphere is the second layer among them. Well, it is a gaseous layer in red color and is about 3000-5000 kilometers in depth. But its red color is only visible during eclipses and this layer locates between the photosphere and solar transition region.
Chromatography: The laboratory and scientific procedure useful for separating the mixtures. It works by sending it into a liquid content with the help of a medium that has components moving at not the same rates.
Chromate Compound: The chemical substances that consist of chromate anions that have the chemical formula CrO42- are chromate compounds. By nature, these compounds are weak bases, salts, and good oxidizers.
Cholesteric: It is the pattern of the liquid crystal where molecules of it are set in parallel rows and maintain a slight rotation effect between the two layers above and below. As the liquid maintains a helical structure, it is chiral in nature.
Chromate: It is a management process of creating a defensive layer of chromate compound by putting chromium compound solution to the metal surface. The main purpose of chromate treatment is to build up the corrosion resistance of base metals. Chemically, it is a good oxidising agent plus anion compound with molecular formula CrO42-.
Chromite: The chromium and iron oxide of chromium is chromite with the chemical formula FeCr₂O₄. it is basically a mineral have its place in the spinel group. Examples of chromite: CrO2– and [Cr(OH)6]3-
Chromophore: Chromophore is a part of any substance or molecule whose presence adds color to it. Fabric conditioners, lycopene, anthocyanins, β-carotene, and pH indicators are a few of the chromophore substances.
Chromium: The transition metal chemical element that holds atomic number 24 with the symbol ‘Cr’ is chromium.
Circuit: It is an electric circular path where free electrons flow in a closed space and help in the production of current. Transistors, capacitors, resistors, etc. are examples of circuits.
Cobalt: The chemical element with atomic number 27 and holds the Co symbol in the periodic table is cobalt. It is generally available in Earth’s crust in the chemical combination form, especially in the naturally occurring meteoric iron. Cobalt is useful in making batteries, and magnetic alloys.
Cohesion: The force of attraction that drags the particles of the same substance and keeps them bonded is cohesion. It also measures the rate of molecules bonding or grouping with the other molecules.
Collagen: Collagen is the soft tissue present in between the cells of humans and mammals. It consists of proteins made of amino acids. It helps in maintaining healthy skin in humans.
Colloid: A colloid is a substance whose particles are larger than atoms or molecules, even though not visible to human naked eyes. Basically, it is homogenous in nature and the particles do not settle down like milk, paint, butter, smoke, etc.
Combined Gas Law: As the name suggests, it combines all the three significant gas laws i.e. Boyle’s law, Charle’s law, and Gay-Lussac’s Law.
PV/T = k; Where P-Pressure, V=Volume, T=Temperature, k=Constant
Columbium: The old name of Niobium is columbium which has the atomic number 41 and the symbol Nb. It is a pure transition chemical element and metal that appears in crystalline form and light grey in color.
Combustion: It is a process of an exothermic chemical reaction between the oxidant molecules and fuel substances. Generally, these chemical reactions release light and heat in the form of flame. A combustion reaction is a reaction where new products are formed along with heat by reacting a compound with oxidant.
If the reaction completely consumes all the fuel reactants then, it is a complete combustion reaction.
Common Name: Common name is the old and historic name of a compound. For example, Baking Soda is the old historic name for sodium bicarbonate.
Common-Ion Effect: It is an effect that determines the subsiding result of electrolyte ionization.
Complex Ion: The ions which take the center position and bond to more than one molecule or ion are complexions. Examples of complexions: Cu(NH3)62+, copper amine ion
Compounds: A compound is a chemical substance that contains two or more chemical elements and atoms. If a substance contains only one element in an atom, then it is not a compound.
Comproportionation: A chemical reaction between reactants that contain the same number of elements and unidentical oxidation number is Comproportionation. The outcome of this reaction is it attains the products that show same oxidation number. The other name for comproportionation is symproportionation.
Concentration: Concentration is the quantity of a material calculated in regard to its weight per volume. If a substance occupies a mixture solution in large amounts, then the mixture is highly concentrated.
Condensation: Condensation is the natural phenomenon of changes happening in the physical matter i.e. from a gaseous state to a liquid state. Simply, the conversion of gas into a liquid is condensation.
The chemical reactions that produce liquid products such as anhydrides from the reactants are the condensation reaction. And the polymer formed from the reaction between two monomers is the condensation polymer.
Conductor: A conductor is a substance that allows electrically charged particles or electrons to flow in more than one direction. Generally, metals are the common electrical conductors. Mostly, we use the word conductors in physical and electrical engineering.
Conformation: The process of attaining different shapes by a molecule because of the rotational movements happening between the groups of atoms around its single bond. A conformer is an isomer that is different from another isomer due to the revolving changes happening in a particle around the single bond.
Congener: In beverages industry, a congener is a by-product of fermentation process while producing different types of alcohol varieties. These materials are the minor chemicals containing compounds such as acetone, methanol, aldehydes, esters, etc.
Conjugate: Conjugate means ‘join together’ in chemistry. That means conjugate is a substance, resultant of a reaction between two or more chemical compounds.
Conservation of Energy: It is the principle in chemistry and physics stating that the energy of reactants (either particles or molecules in a chemical reaction) stays stable in a closed system. For Example Potential and Kinetic Energy: when you make a pendulum swing, the potential energy converts into kinetic energy and always remains stable.
Conjugated System: A system in chemistry in which p-orbitals of delocalised electrons tend to change the single and multiple bonds alternatively. And this drops the whole molecular energy and at the same time rises the stability. Carbo cations, singular pairs, radicals, and even d-orbitals in bigger atoms play important role in the conjugated system.
Conservation of Mass: It is again an important principle in chemistry and physics which states that the mass of reactants and mass of the products after chemical reaction remains the same.
Constant Variable: A constant variable reveals a fixed value which does not change throughout the normal execution of an experiment. For Example, Pressure is a variable in a particular experiment, if so, then the value of pressure i.e. variable does not change during the program.
Convection: Convection is the chief element in weather technology because it is the process of moving energy of heat according to the movement of heated particles. In Chemistry, when the liquid fluids heat up, the heated elements tend to move faster and rise as it becomes less dense. Whereas the cooler molecules or particles drops down consequently which is denser.
Continuous Spectrum: A continuous spectrum of emission that encompasses various wavelengths of different colors is a continuous spectrum. As the name suggests, it strictly does not maintain any gaps between the wavelengths. Emission lines are the lines in the continuous emission spectrum.
Contributing Structure: It is the basic structure of Lewis’s structure from a resonance set that helps to explain the delocalized electrons.
Coordination Bond: Coordination bond is the dative bond or coordinate covalent bond or dipolar bond, exists between two atoms where one of the atoms share electrons to form the bond.
Coordination Number: The specific number of atoms bonded to another specific atom is a coordination number. For Example, 4 is the coordination number of carbon atoms in a Methane molecule because it has four connected hydrogen bonds.
Copernicium: Coperniciumis one of the main transition elements which are metal in nature with the symbol ‘Cn’ and atomic number 112. Unumbium is the earlier name of copernicium with the symbol ‘Uub’.
Coordination Compound: A compound is represented as a coordination compound when it consists of at least one or more than one coordinate bonds. For instance: most of the metal complexes as well as mixtures without alloys such as hemoglobin.
Copolymer: When a polymer prepared of using monomer species is a copolymer and the process is co-polymerization. If the copolymers consist of two monomer species, then it is a biopolymer.
Copper: Copper is a chemical and metal element that is soft and ductile showing high electrical and thermal flow of electricity. Copper has the atomic number 29 and the symbol ‘Cu’.
Corrosion: Corrosion is a gradual destruction of a metal substance into its stable form i.e. oxide, sulphide, and hydroxide. The process is natural, irreversible, gradual, and chemical because it happens through chemical or electrochemical reactions in the environment. Well, this process can damage even the living tissues.
Corrosive: The substance that is capable of damaging something like living tissues or materials completely through touching base is corrosive. For instance: strong bases and acids are the best examples of corrosives.
Coulomb’s Law: Coulomb’s law, otherwise known as Coulomb’s inverse square law states the quantity of force that exists between any two inactive and electrically charged electrons. And the electric force between those charged electrons is Coulomb force or electrostatic force.
The formula is: F ∝ Q1Q2/r2
F=Force between charges, Q’s represent Quantity of electric charge, r= Distance between two charges.
Coulomb: It is the International System of electric charge units. It is equal to the amount of electricity that is transferable by one ampere electric current per second. It holds the symbol ‘C’. And an electron carries a charge of -1.6 x 10-19 coulombs.
Covalent Bond: The chemical bond formed by sharing of electrons between the two or more reactants i.e. atoms or chemical compounds is a covalent bond. Well, the bond forms only when the reactants participate in a chemical reaction with the help of attractive and repulsive forces.
Covalent Compounds: The chemical compounds formed during the occurrence of covalent bonding by sharing valence electrons are covalent compounds. Ex: DNA, sucrose, and water etc.
Cracking: The process of breaking of covalent bonds existing in the larger hydrocarbons to form smaller hydrocarbons is cracking.
Covalent Radius: The half of the radius between the nuclei of two single-bonded homo-nuclear atoms is the covalent radius. It helps in stating the size of the atom that takes part in forming a covalent bond with another atom. Pico meters or angstroms are the measuring units of covalent radius. By definition, the total addition of two covalent radii makes the exact length of a covalent bond.
Crenation: The method of describing an object or cell’s reaction to hypertonic solution is crenation. This process is also useful in describing the shape of an object. In zoology and biology, the same process is helpful in deciding the shape of things. For example; a plant’s leaf or shell and an animal cell etc.
Critical Mass: Critical mass is the smaller quantity of fissile substance useful for maintaining a nuclear chain reaction.
Critical Point: Critical point is the point at which two substances start to get indistinguishable from each other. And the pressure created at that point is critical pressure while the critical temperature is the temperature at the critical point. On the other hand, one mole of chemical compound generated at the critical temperature and pressure is critical molar volume.
Cross-Link: Cross-Link is the link useful for the formation of connections between polymers with each other.
Cryogenics: Cryogenics is the study of physics that deals with the materials or chemical reaction products existing at very low temperatures. It is widely useful in a variety of fields such as rockets, MRI devices, etc.
Cryogenic Gas: Cryogenic gases are nothing but the vapors of cryogenic liquids when set to heat at very low temperatures. Liquid nitrogen is the best example of cryogenic gas.
Crude Oil: Crude oils are petroleum products that occur naturally underneath the Earth’s crust during geological changes. For example Petrol, gasoline, etc. These petroleum products use organic materials and hydrocarbon deposits to produce petrochemicals.
Crown Ether: The chemical compounds that contain a lot many ether groups in the form of ring-like structures are crown ethers. These bring certain cations together by bonding and forming complexes. Cyclic oligomers of ethylene oxide are the most common examples of crown ethers. Also, these are useful in promoting chemical reactions by turning inorganic catalysts into organic compounds.
Crystal: Crystal is a crystal-like hard substance that is homogenous in nature and contains natural symmetrically arranged structures. These structures results in the formation of crystal lattice which is capable of extending in all instructions. On the other hand, any highly transparent object made of glass and also contains high refractive index is a crystal. Ex: Rock Candy, Quartz, etc.
Crystallization: The process of formation of hard solid-like substances in which atoms or molecules organized in a symmetric structure is the crystallization process. Precipitation and freezing are the most common methods of the crystallization process.
Crystal Field Splitting: The change in between the energy of d-orbitals, which helps in describing the wavelength differences of colors of similar ligand complexes is crystal field splitting.
Crystallization Water: When water molecules are bound in a specific crystal-like structure, then it is the crystallization of water. Hydrates, water-containing, and crystalline chemical compounds crystal salts are the crystal salts and the best example of crystallization of water.
Crystals of Silver: These are the metal crystals and the term which denotes the chemical compound name i.e. AgNO3, silver nitrate.
Cupric, Cuprous, and Cuprum: The chemical compounds which contain copper in a +2 oxidation state are cupric compounds. Also, this is the term for copper (II) ions. Well, cuprous is the term for copper (I) ions and the compounds that contain copper in +1 oxidation state are cuprous compounds. Cuprum holds the symbol ‘Cu’ and is the Latin name for copper in periodic table.
Cubic Centimetre: It is the unit of volume that represents the cube volume of 1 mm i.e. 1 cm x 1 cm × 1 cm. cc or cm3 denotes the cubic centimeter.
Curie Point: Curie point is a temperature point at which magnetic materials change or lose their magnetic properties. Whereas the non-SI unit of radioactivity is just simply a ‘Curie’ which denotes by Ci and 1 Ci= 3.7 x 1010 disintegrations per second.
Current: The continuous stream of electric charges in a complete circuit system basically represents current. Ampere is the unit of current! 1 ampere = 1 coulomb per second!
Cycloalkane and Cycloalkene: The alkane compound that is in a ring-like structure with carbon-carbon bonds is a cycloalkane. Whereas Cycloalkene is an alkene compound with ring-like carbon atoms in a bond.
Curium: The chemical composition and radiation-emitting metal of actinide sequences holding the atomic number 96 is curium. It is a man-made element as it does not occur naturally. And it occurs when plutonium ions are bombarded with helium ions.
Cyanide: The harmful chemical compound consisting of carbon and nitrogen atoms in various forms is cyanide, i.e. HCN or CNCl or in combination with potassium (KCN) and sodium (NaCN). Also, the compounds containing the C≡N group were determined as cyanides. These are the harmful and fast-acting chemical compounds that even lead to death of living organisms.
Cyanate: Cyanate is an anion that contains the functional group ‘-O-C≡N’. Also, cyanate refers to the salts or ester groups of cyanic acid.
Cystine: Cystine is the water-soluble white solid that serves the most important biological functions such as mechanical linkage of proteins and redox reactions. Chemically, it holds the molecular formula (SCH₂CH(NH₂)CO₂H)₂ which represents the oxidised dimer form of cysteine amino acid.
Cysteine: The naturally occurring protein genic amino acid is categorized by a side link group i.e. –SH group is Cysteine. It holds the chemical formula: HOOC-CH–CH₂-SH. This compound helps in maintaining protein structures in the human body and helps in producing another amino acid, Taurine. The shortened form of cysteine is ‘Cys’ or ‘C’.
Cuvette: The most important laboratory equipment used in chemical labs for holding liquid samples. This equipment is a straight and tube-like transparent container, made out of glass or quartz, or clear plastic.
Carbon Cycle: The continuous movement of carbon atoms around the Earth’s atmosphere is the carbon cycle.
Centripetal Force: The force acting on an object to make it move in a circular motion is centripetal force.
Collision: Collision is the condition where two or more substances interact to alter their energies and momentums.
Coefficient: It is a value that represents the number of reactant molecules involved in the chemical reaction to produce a particular number of product molecules.
Compound machine: The machine that combines more than two simple machines is a compound machine.
Biology Words Starting with C
Capillary: In biology, capillaries are the small blood vessels useful to make linking between arteries and veins. The main function of these blood vessels is to carry nutrients and oxygen to the single cells existing in the whole body. Also, they does exchange of substances between tissues and blood.
Cardiac Muscle: The muscle that is present around and inside the heart of living organisms to perform blood pumping and coordinated contractions is cardiac muscle. It is one of the three main body tissues, striated and functions involuntarily.
Carnivore: Carnivore means nothing but the animals or mammals that depend on other animal’s flesh or meat. Carnivores, otherwise known as meat-eaters get their food by hunting or scavenging other animals.
Cell membrane: Each and every individual living cell in living organisms including humans consists of a thin outer layer which is nothing but ‘Cell Membrane’. The primary function of this membrane is to protect the inside content of living cells from the environment toxins.
Carrying Capacity: The fairly accurate value of a species population size in a specific habitation is carrying capacity.
Cellular Respiration: This is a metabolic process in living cells that transforms the chemical energy into life-sustaining daily substances through chemical reactions. These life-sustaining substances may include adenosine triphosphate, water, carbon dioxide, and other waste products.
Chemical Weathering: Weathering is the naturally occurring method of breaking down the rocks, and soil minerals. While chemical weathering is again the natural method of acidic rainwater getting to react with minerals in the soils and rocks. This reaction results in formation of new salts and minerals that are soluble. And the reaction is hydrolysis.
Central Nervous System: Central nervous system constitutes the brain and spinal cord which together helps in controlling body and mind functions and responses. This system works with other nervous system in the body with the help of electrical signals existing in the neurons.
Childhood: Childhood refers to the stage or phase of being child i.e. the age before the children attain their teenage. During this stage, children go to school, play around, and communicate with the people around them and turn into strong and confident teenagers.
Chloroplast: Chloroplasts is the plant and algae cell structure that helps in absorbing light and making sugars with the help of green pigment, chlorophyll. And the process of absorbing light and making food is photosynthesis.
Circulatory System: The system of blood vessels that carry blood from and to the heart is circulatory system. The other names of this organ system is cardiovascular or vascular system. This system not only pumps blood but also transports oxygen, nutrients, and other substances like hormones to living cells.
Classification: Systematic grouping of animals and plants into taxonomic groups based on their similar and dissimilar characteristics is classification.
Cleavage: In biology, cleavage is the process of cell division in its early embryo stage i.e. in zygote stage. While in chemistry, it means the breaking characteristic of minerals from end to end along smooth surfaces.
Climate: The typical and long-standing weather conditions i.e. over a period of hundred to thousand years in a particular area is climate. Weather conditions change within hours or minutes but climatic conditions do not change very often. The amount and timing of precipitate formation, weather extremes, freezing points, wind speeds and directions, amount of sunshine, and local geography plays important role in deciding an areas climate.
Climate Zone: The zone that describes the climate of different areas that commonly show the same weather conditions is climate zone.
Climax community: Climax community is the stable stage of different communities such as plants, animals, and other living species. Beyond this stage, there is no continuation of ecological successions.
Cnidarian: Cnidaria is the phylum belonging to the Animalia family which contains aquatic animals from marine and freshwaters. Cnidarian is one of the aquatic animal, also called coelenterate from this phylum and family. Ex: Jelly fish, hydras, corals, etc.
Cold front: Cold Front is a zone where a mass of cold air occupies the mass of warm air in that particular area and also maintains low pressures.
Comet: The icy, gaseous, and kind of small solar system constituting frozen gases, rock, and dust is a comet. These emitting content of comet happens when it reaches the orbit of Sun because it gets warm up and stats releasing.
Commensalism: Commensalism is a habitual process of communication between two living organisms through which one organism benefits from another. But without harming the other opposite organism or any other living thing.
Community: A group of living organisms that share the same habitat in regards to climate, food, shelter, etc. is a community.
Compact bone: Compact bone is the dense bone that encompasses organic and inorganic salts forming the tough outer layer of the bone.
Competition: The process of attaining a sort of interaction between a group of living organisms including humans to share same food and other resources is competition.
Compound Microscope: The microscope that uses at least two or multiple lenses is the compound microscope. It helps in enlarging the sample images in the laboratory and gives good observation results.
Scientific Terms & Instruments List on Letter ‘C’
Computer: The electronic device that helps in storing and processing the digital information based on the instructions and in the binary form is computer.
Concave: A curved and rounded inward side of an object is concave. For example: the inside part of the spoon.
Coniferous: Any green tree or shrub with needle-like structured leaves and produce cones is a coniferous tree. The common coniferous plants are Pinophyta or Coniferophyta.
Conservation: The process of preventing natural resource waste from extinction and restoration practices is conservation.
Constellation: A specific area in celestial sphere consists of visible stars that form the outline patterns resembling a particular animal or creature or other historic persons is constellation.
Consumer: In a food chain, consumer is the living thing who/that finds food and energy by consuming other living things. Heterotrophs is the other name for consumers.
Continental climate: Continental climate constitutes the comparatively a dry climate, mostly seen in Asia and North American regions. It reveals very high cold and dry temperatures along with significant annual variation.
And the place where two continental crush plates crash together along their boundary of them is a continental-continental collision.
Continental drift: It is the best way to describe the movement of continents over a long period of time by geologists.
Coral reef: The limestone deposits of corals (tiny aquatic organisms) that made up the underground ecosystem are coral reefs.
Corona: Corona, a newly discovered virus that causes an infectious disease known as COVID-19. Covid infection is the infection that infect sinuses, nose, throat, and in respiratory organs. On the other hand, the corona is the external cover of Sun’s atmosphere.
Cornea: Cornea is the external membrane of eye, which not only helps to cover the eye but also helps in giving clear view of any object by focusing light.
Cytokinesis: The physical and division process of living cell, through which parent cells gives birth to two daughter cells is cytokinesis. Cytokinesis is a process that occurs along with two other divisional processes i.e. mitosis and meiosis.
Cytoplasm: The thick filling substance that exists in living cells in order to perform all the life-sustaining processes is cytoplasm. It covers with a cell membrane to avoid leakage from inside of the cell. And this fluid is rich in salts, proteins, and water content.
Carbon Dioxide: It is a colorless gas that plays the main role in transporting oxygen to the other parts of the body. Plants utilise carbon dioxide to prepare food by themselves whereas humans breathe out this gas to purify the circulatory system.
Carbon Dioxide Fertilization: The process of increased growth of any variety of plant species by providing carbon dioxide in higher quantities is carbon dioxide fertilization.
Carbon Monoxide: The distasteful, fragrance-free, colorless, and combustible gas produced from the combustion of charcoal, fuel, gasoline, wood, and other fuel materials. In the human body, this gas helps in pumping oxygen to all other parts of the body.
Catabolism: A metabolic process helpful in breaking down the larger molecules into smaller units and produce energy is catabolism. This process can happen either by oxidation or along with other anabolic reactions.
Celestial Body: Also known as heavenly body is a naturally occurring solid object in space. These celestial bodies are clearly visible in the sky from Earth such as Sun, Moon, Stars, and Planets.
Chyme: Chyme is the thick and semi-solid substance present in the stomach after mixing up with digestive gland secretions. It is thrown out of a person’s stomach into the small intestine for further digestion.
Co-Enzymes: Co-Enzymes is the non-protein substances that help in activating enzymes and make them act as a catalyst during biological processes. Chemically, co-enzymes bind to the protein molecules in order to make an inactive enzyme active. S-adenosyl methionine and B-vitamins are the best examples of co-enzymes.
You may be interested to read
Science words starting with ‘F’
Science words starting with ‘k’
Science words starting with ‘G’
Science words starting with ‘I’
Science words starting with ‘Q’
Science words starting with ‘A’
Science words starting with ‘B’
Science words starting with ‘Z’