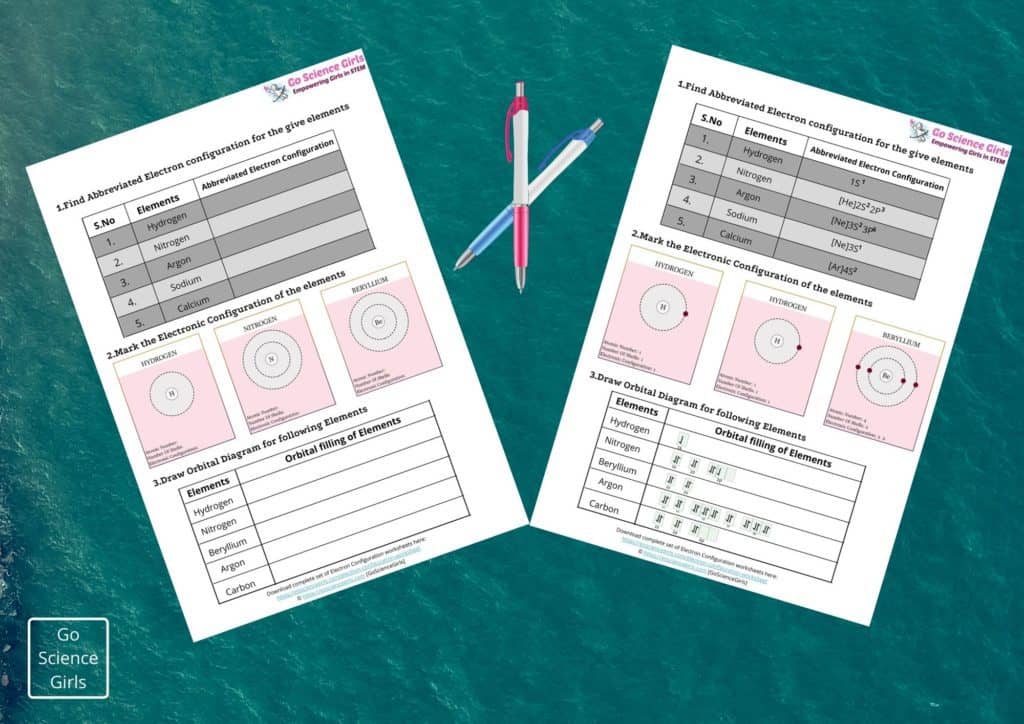
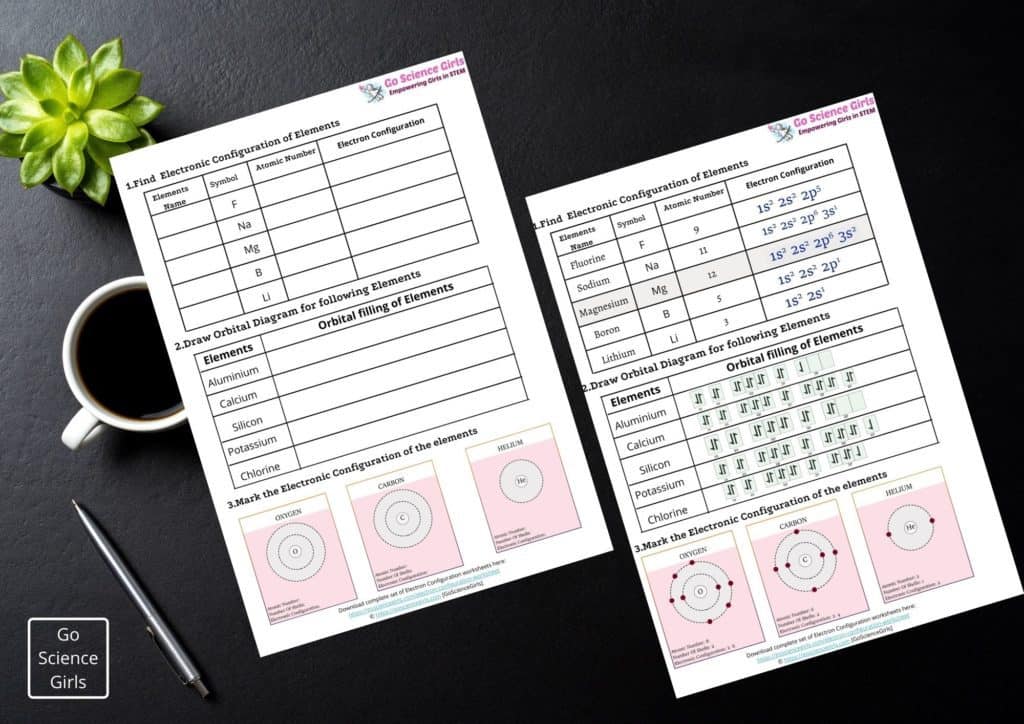
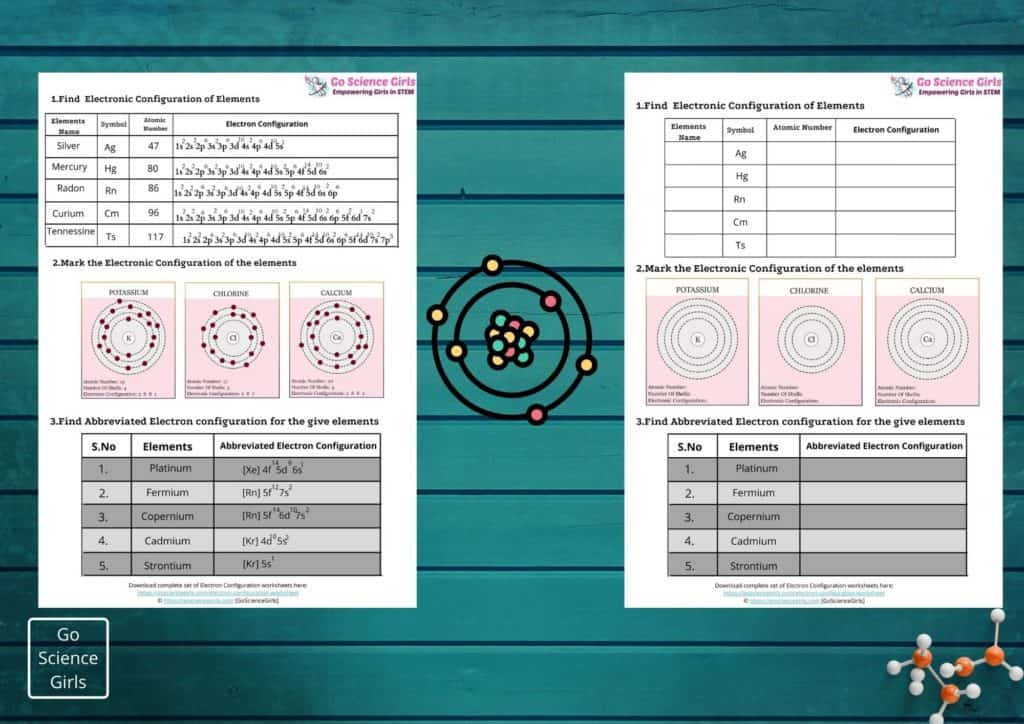
Let us learn more about the electronic configuration along with some awesome worksheets and orbital diagrams in this article.
Definition and Basics of Electronic Configuration
The electron configuration of an element is a standard representation of its electron arrangement in the orbitals of its atom. This notation also helps in understanding the bonding capacity of electrons in an atom through magnetic and other chemical features.
Well, using the periodic table, anyone can easily write the electronic configuration of any element. For Example: The electronic configuration of Potassium is 1s22s22p63s23p64s1
The letters in the electronic configuration of any element i.e. s, p, d, and f represent the four different atomic orbitals.
Well, atomic orbitals are nothing but the energy quantum states that tell the uncertain behavior and exact location of an electron in the electron cloud.
These four atomic orbitals are present around the nucleus of an atom and represent different energy states.
By studying these atomic orbitals, scientists calculate and write the location and energy state of an electron plus its interaction in the atom to create chemical bonding.

You might have observed the standardized notation while writing electron configuration. This notation follows the following pattern: The type of energy level and orbital are written as the first step, for ex: 1s. Then, the number of electrons located in each orbital is denoted in the superscript of the orbital symbol i.e. 1s2.
Detailed Explanation of Atomic Orbitals
As we all already know, electrons bear charge i.e. either negative or positive, and are free to change their locations often. Their movement from one energy state to another completely depends on the attractive and repulsive forces between the positive and negative charges.
Well, positively charged electrons get attracted by negatively charged electrons while likely charged electrons repel each other.
And because of these repulsive forces among the likely charged electrons, the electrons scatter in different patterns around the nucleus of an atom.
These wonderful outlines of geometrical positioning of electrons represent different states around the nucleus called atomic orbitals.
That is the reason, we observe four different atomic orbitals around the nucleus of an atom. I.e. s, p, d, and f atomic orbitals.
S— Sharp
P—Theory
D—Diffuse
F—Fundamental
To determine the electronic configuration of an element, one must follow three important principles from quantum mechanics.
These theorems include Aufbau Principle, Hund’s Rule, and Pauli Exclusion Principle—which forms the set of general rules to write electronic configuration for any element in the periodic table. Let us see how and in what are those rules:
Aufbau Principle
Aufbau is a German term and it says ‘Building Up’! Well, the principle of Aufbau denotes that electrons occupy energy states in the increasing order form. That means, they occupy the lowest energy state in the beginning and continue to the next highest energy level and go on…
Have a look at the order of electron occupying energy states in its atomic orbitals:
1s<2s<2p<3s<3p<4s<3d<4p<5s<4d<5p<6s<4f<5d<6p<7s<5f<6d<7p
With reference to the above order of occupation, it is clear that electrons will not occupy the highest energy orbitals until they already filled up the lowest energy orbitals. For ex: 7s, 5f, 6d and 7p subshells will not be filled up without the occupation of electrons in 1s to 6p subshells.
Note to remember: The electrons prefer to occupy the lowest orbital, 4s first rather than the still lowest 3d orbital, against the rule. This is because the electrons in 3d orbital repel strongly as they are very close to the nucleus of the atom.
Consider Bromine element located in the Group VII, Period 4 of the periodic table. It has 35 electrons and among which 7 electrons are valence electrons.
Two electrons out of 7 valence electrons occupy 4s orbital first and the rest occupy 4p orbital. And hence the electronic configuration of bromine atom is 1s22s22p63s23p64s23d104p5, satisfying Aufbau principle.
Hund’s Rule
Hund’s rule denotes that electrons must occupy every single orbital of a subshell with at least one electron with same spin direction. And then they can start double occupying of orbitals of subshell.
However, Hund’s rule strictly follows the theory of atomic spectra. Atomic spectra is nothing but a theory that represents the ground state of an atom using open electronic shells.
Pauli Exclusion Principle
According to Pauli Exclusion Principle, two or more electrons of a single atom cannot occupy the same quantum state and possess the same quantum values.
To put it simply, every individual electron encompasses of four quantum numbers and two electrons must exhibit opposite spins when located in the same orbital.
Let us learn what Quantum Numbers and Spin values of an electron are!
The Denotation of Quantum Numbers
In quantum physics and chemistry, quantum numbers play a major role in denoting the locality and energy values of an electron in its atomic orbital. Basically, quantum numbers represent number values in the quantum system in the form of four quantum numbers. They include:
1) Principal Quantum Number (n)
2) Orbital Angular Momentum Quantum Number (l)
3) Magnetic Quantum Number (ml)
4) Spin Magnetic Quantum Number (ms)
Principal Quantum Number (n)
As the name proposes, ‘n’ is the chief energy level where the electron is easily detectable. And the ‘n’ value is determined based on the distance of energy level from the nucleus of the atom. These values range start from 1 to n…, while n denotes the value of the outermost shell occupied with electron.
For example: If the principal quantum number is n=1, then it confirms that the electron is positioning closer to the nucleus. In the same way if n=2, 3,4,5,6 and go on…the electron location is farther away from the nucleus.
Let us consider the Iodine element: the outermost electrons of the Iodine atom located in the 5p orbital. So, the principal quantum number of Iodine is 5. The same method implies to every individual atom of the periodic table.
Angular Momentum Quantum Number (ℓ)
This quantum number is otherwise popular as orbital quantum number. The main purpose of angular quantum number is to denote the orbital shape and the type of subshell of an electron occupies.
The ‘ℓ’ values remains between zero and n-1 while depending on the values of principal quantum number. Here, if the n value is 2, then the ‘ℓ’ value is either 0 or 1.
For ex: If the ‘ℓ’ value is 0, then it represents the s orbital; ℓ = 1, then it is p orbital; ℓ = 2, it is f orbital and if ℓ = 3, it is f orbital.
In atomic theory, the angular quantum number plays an important role since it signifies the magnitude of the shape of atomic orbitals and its impact on chemical bonding of electrons.
Magnetic Quantum Number (ml)
Magnetic quantum number, denotes the alignment of given subshells in the air and produces the ℓ value through definite axis. For ex: The three dimensional axis of a nucleus of atom denoted by X, Y, and Z axis in three dimensional space. And the electrons can locate in this three dimensional space of a nucleus.
The formula that derives the value of magnetic quantum number is ml = (2ℓ + 1)! Where ℓ = angular quantum number. So, overall values of quantum numbers based on this formula could be;
If n = 2;
ℓ = 0 0r 1;
For ℓ = 0; m1 = 0 and For ℓ = 1; m1 = -1, 0, +1
Spin Magnetic Quantum Number (ms)
Every individual electron is free to spin in either of the two associated ways i.e. +1/2 and -1/2 spin. So, the role of spin magnetic quantum number is to identify the type of spin an electron is undergoing in its orbital.
And these spins of the electrons are also denoted by upward ↑ and downward arrows ↓. Since the electrons spin, there is the production of magnetic field.
According to the rules of electronic configuration, two electrons can locate in the same orbital but with opposite spin directions. If the value of ms is +1/2 for an electron, then that electron is ‘alpha electron’ while the electron with -1/2 spin value is ‘beta electron’.
Find the table representation of possible subshells based on the principal energy levels below: Here we have included the values of n up to 4 and the rest of the values follows the same method.
| Principal Quantum Number Value | Number of possible sublevels | Possible Angular Momentum Quantum Numbers Value | Electronic Configuration from ‘n’ values and Sublevels |
| n = 1 | 1 | l = 0 | 1s |
| n = 2 | 2 | l = 0 | 2s |
| l = 1 | 2p | ||
| n = 3 | 3 | l = 0 | 3s |
| l = 1 | 3p | ||
| l = 2 | 3d | ||
| n = 4 | 4 | l = 0 | 4s |
| l = 1 | 4p | ||
| l = 2 | 4d | ||
| l = 3 | 4f |
Since the orbital quantum number values is less than the principal quantum numbers, there is no existence of 1p, 2d and 3f atomic orbitals.
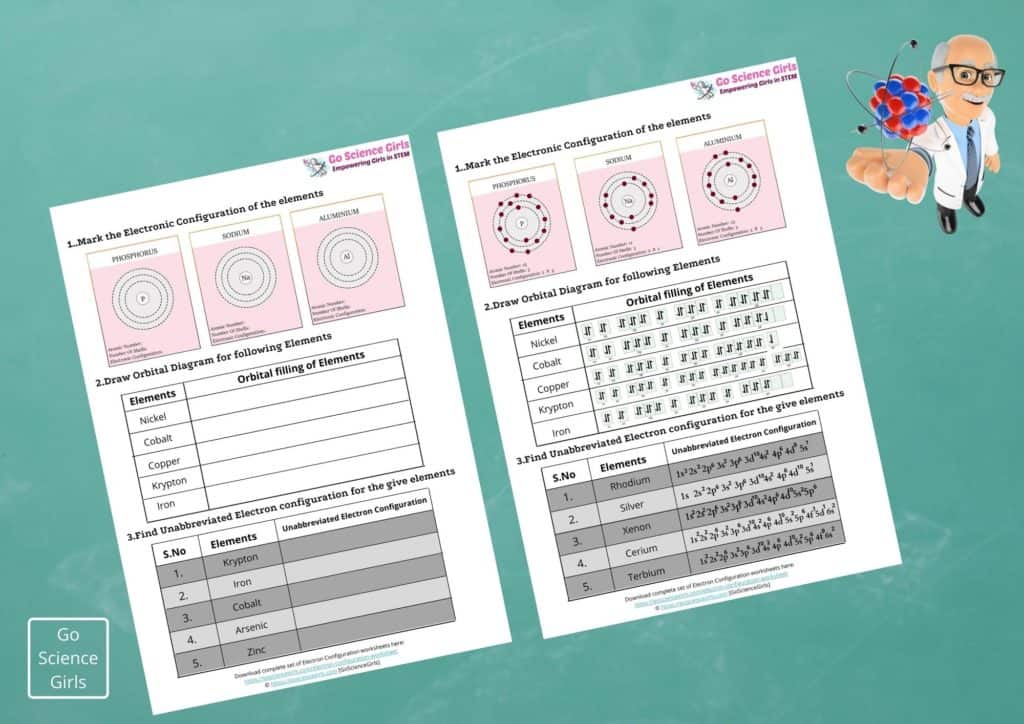
Electron Configuration Orbital Diagram

Electron Configuration of any element only reveals about the electron distribution among atomic orbitals around the nucleus of an atom. Whereas orbital diagram is an illustrative representation of location and spin of the electrons within the orbitals in the form of arrows.
Therefore, we can say that the transcribed description of orbital diagram is nothing but electron configuration.
So with the help of orbital diagram, we can easily find out which type of atomic orbitals filled out and which are partially occupied with electrons.
In this diagrammatical representation, arrows represent electrons and its point of direction represent the spin of the electron. Let us see one example of orbital diagram:
Electron Configuration of Nitrogen: 1s2 2s2 2p3
Orbital Diagram for Nitrogen:

S, P, D and F are the four different atomic orbitals located around the nucleus of an atom with different energy levels. Electrons fill up these orbitals in an order and here is the cheat sheet that helps you better understand the order of orbital diagrams.
And the three rules that help in generating orbital diagram are Hund’s rule, Aufbau principle and Pauli Exclusion Principle.

Ground State vs Excited State Electron Configuration
At this point, we all are aware of that an electron’s location is uncertain and only reveal their probability of exact location around the nucleus.
Keeping the uncertain behaviour of electrons in mind, our scientists discovered different energy levels around the nucleus of an atom. And also said that these atomic orbitals encompasses of electrons at highest possibility.
Well, the other basic information about these energy levels include: The atomic orbitals that are close to the nucleus of an atom exhibits lower energies while the farther ones exhibits higher energies.
Based on this information, let us learn about ground and excited state levels and also about the differences between these two states of energy levels.
Ground State: The lowest possible energy levels among all the atomic orbitals around the nucleus possessing electrons refers to ground state. The ground state electron configuration is the most stable one that means it possess stable arrangement of electrons. The other name for ground state is vacuum state.
Excited State: The highest possible energy levels among all the atomic orbitals around the nucleusrefers to excited state. They possess electrons with unstable arrangement and hence the electrons excite to jump from excited to ground state often.
Differences between Ground State and Excited State
| Ground State | Excited State | |
| Definition | Electrons locate in lowest possible energy levels | Electrons locate in highest possible energy levels |
| Stability | Highly Stable | Highly Unstable |
| Lifetime | Very Long Lifetime | Very Short Lifetime |
| Energy | Very Less Energy or sometimes with Zero Energy | High Energy |
| Electron Location | Electron Location is always intact to lowest possible energy levels | Electron Location is always intact to highest possible energy levels |
| Distance From Atomic Nucleus | Distance between atomic nucleus and ground state electron is very less | Distance between atomic nucleus and excited state electron is comparatively high |
Electron Dot Configuration
To understand better about electron dot configuration, we need to learn a couple of terminology related to electron configurations. Here we go:
Valence Electrons
The electrons of an atom present in its outermost shell or energy level that are useful for forming chemical bonds are valence electrons.
Hence, finding out valence electrons for an atom is very important in order to learn the particular atom’s reactivity. For example: The valence electrons of oxygen atom are six; out of which two are present in 2s subshell while the rest four are present in 2p subshell.
Finding Valence Electrons: Non-Transition Metals
1) Pick a periodic table where you will find all the variety of elements in the boxes. And learn about periodic table terminology like rows, columns, periods and groups.
2) Choose any element of your choice from the periodic table.
3) Recognise the group numbers and memorise them.
4) That’s it! The digit on the ones place of the group number refers to the number of valence electrons of an element.
Finding Valence Electrons: Transition Metals
Transition metals does not have traditional valence electrons. Hence, we cannot predict the number of valence electrons of a transition metal with certain number. Below is the possibility of number of valence electrons of transition metals based on group number.
Group 3: Possibility of 3 valence electrons
Group 4: Possibility of 2-4 valence electrons
Group 5: Possibility of 2-5 valence electrons
Group 6: Possibility of 2-6 valence electrons
Group 7: Possibility of 2-7 valence electrons
Group 8: Possibility of 2-3 valence electrons
Group 9: Possibility of 2-3 valence electrons
Group 10: Possibility of 2-3 valence electrons
Group 11: Possibility of 1 or 2 valence electrons
Group 12: Possibility of 2 valence electrons
For ex: The transition element belonging to group 5 may consists of two to five valence electrons based on the type of situation it is going through.
Finding Valence Electrons with an Electron Configuration

1) Choose an element and write its electronic configuration.
2) Using Octet Rule, arrange the electrons to its orbital shells based on electron configuration.
3) Trace out the number of electrons present in the outer most shell.
4) Make use of periodic table rows and determine orbital shells. Then, determine the valence electrons based on outermost shell electrons and orbital shells.
Core Electrons
The electrons which do not participate in any type of chemical bonding and do not refer to valence electrons are core electrons. These electrons are usually found in inner energy levels and fully occupied and hence referred to chemically inert electrons.
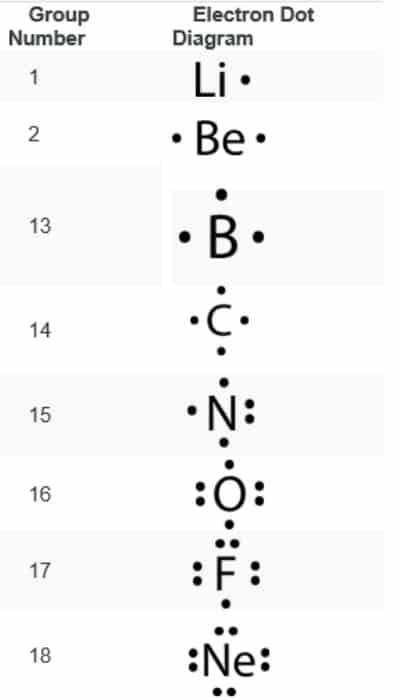
Now, let us learn about Electron Dot Configuration
Electron dot configuration is a type of diagrammatic illustration of number of valence electrons of an element in the form of dots around the element.
The number of dots around the element represent the number of valence electrons of that particular element.
In chemistry, electron dot configuration has its own significance and this representation of valence electrons was invented by American chemist Gilbert Newton Lewis.
Hence, the electron dot configuration is otherwise popular with the names ‘Lewis diagram’ or ‘Lewis structure’ or ‘Lewis Electron Dot Diagram’ in his honour. For Ex:
Calcium—Electron Dot Configuration of Calcium is: .Ca.
Steps to determine the electron dot diagram
1) Analyse the total number of valence electrons of every atom in a molecule.
2) In case of anion molecule, add the extra electrons around the element while drawing dot diagram.
3) In case of cation, subtract the electrons around the element from the total number of valence electrons while drawing the dot diagram.
4) The least possible electronegative atom or ion is placed in the middle of the molecule and connect the atoms using single bonds only.
5) Then, allot the lone pair of electrons to every single atom of a molecule.
6) Check out for every atom whether it possess octet configuration. If any atom does not have octet configuration, then you need to fulfil the octet valence of every individual atom.
7) If necessary, you can transform the lone pair of electrons into bond pair of electrons to fulfil octet rule.
Solving Electron Dot Formula of CO2
- The carbon atom is the central atom of the molecule.
- The oxygen atom consists of 6 valence electrons and 2 lone pairs. So, it can bond to central atom using double bond.
- Carbon atom consists of no lone pair of electrons since it has 4 valence electrons. Therefore, it can bond to oxygen atom using double bond.
Writing electronic configurations for the elements present in the initial periods and groups of the periodic table is easy and simple. But writing electronic configuration of elements in the periodic table that come after noble gas group is lengthy and tedious.
That is where the role of abbreviated and unabbreviated electron configurations come into the picture. Have a look!
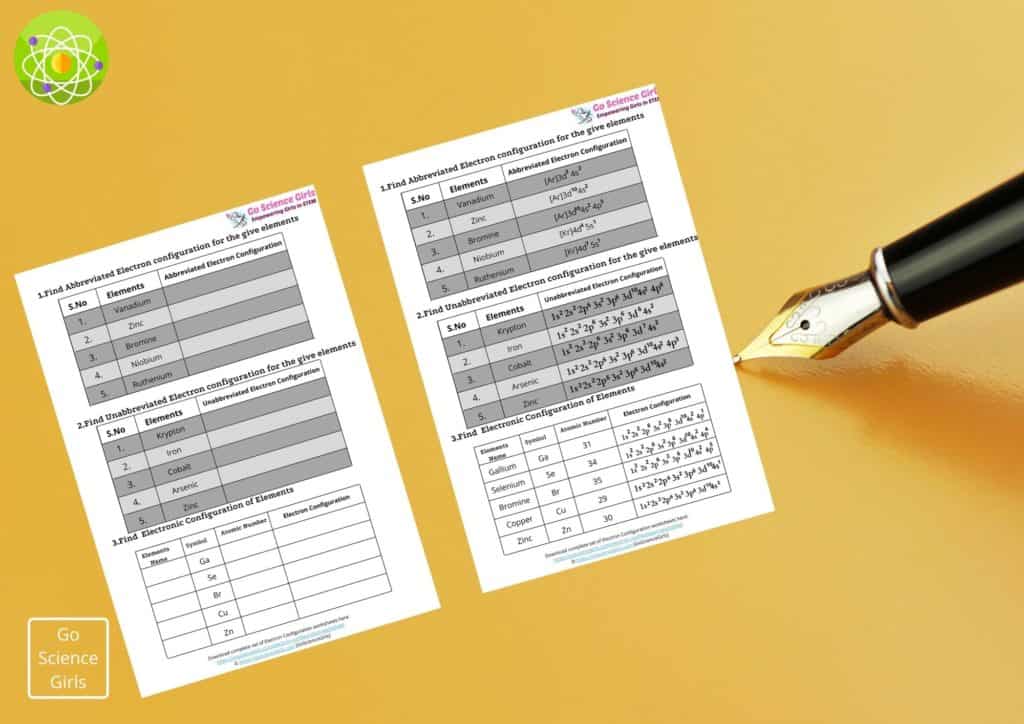
Abbreviated Electron Configuration

Abbreviated electron configuration or Noble gas notation, describes the electronic configuration of elements based on the last column of elements i.e. noble gases. For ex:
The electronic configuration of Neon is 1s2 2s2 2p6 and for Aluminium it is 1s2 2s2 2p6 3s2 3p1
To make it easy and convenience to write, we can write the electronic configuration of Aluminium using noble gas notation as [Ne] 3s2 3p1.
Let us study in detail about this example: Noble gas elements have completely filled subshells and hence the elements with completely filled subshells can replace them.
And while replacing the noble gas element is written in square brackets. In this way, abbreviated electron configuration is much more useful for elements that has higher atmic numbers.
Step by Step Instructions to write Abbreviated Electron Configurations
1) Find out the element symbol using periodic table.
2) As a second step, you need to check for the noble gas element present at the right side of preceding horizontal row. Then, mention it in square brackets. For ex: [Ar] represents the primary 18 electrons of zinc atom while writing its electronic configuration.
3) In the third step, scroll down and back to far left side of periodic table! Then, write the outer electron configuration of your desired element by succeeding the elements from left to right associated with every column.
That’s it! The electronic configuration of zinc atom is [Ar] 4s2 3d10
Unabbreviated Electron Configuration
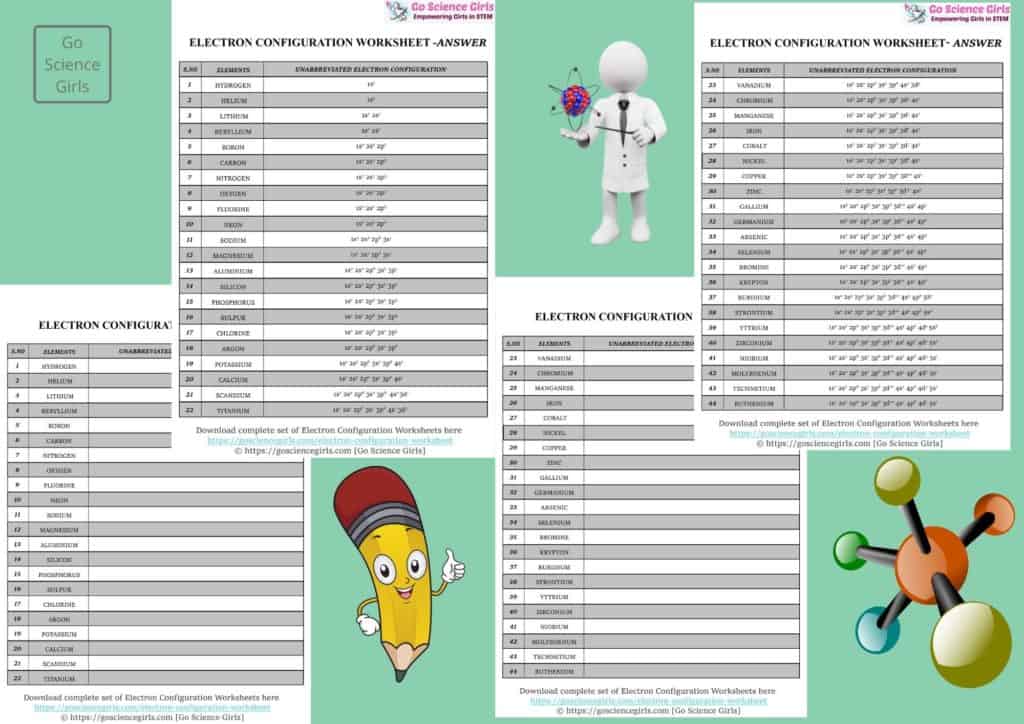
The unabbreviated form of electron configuration is the configuration that does not utilise noble gas notation while writing the electron configuration of elements.
Hence, unabbreviated electron configuration remains much longer, confused and time-taking.
For example: let us learn the abbreviated and unabbreviated form of Gold metal in the periodic table.
The unabbreviated electron configuration of Gold is:
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 while the abbreviated electron configuration of Gold is [Xe] 4f145d106s1
You might have observed that the unabbreviated electron configuration of Gold is long, tedious and takes time to write it completely. Therefore, the noble gas element ‘Xe’ denotes the completely filled outermost shells and becomes [Xe] 4f145d106s1
Complete Electron Configuration for Iodine
Iodine is the stable halogen with atomic number 53 and has symbol ‘I’. The complete electron configuration of Iodine is:
Unabbreviated Electron Configuration: 1s2 2s²2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d10 5s2 5p5
Abbreviated Electron Configuration: [Kr] 4d10 5s2 5p5
Complete Electron Configuration of Zirconium
Zirconium is a strong transition element with atomic number 40 and symbol ‘Zr’. It consists of 40 electrons in total in the shells.
Unabbreviated Electron Configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d2
Abbreviated Electron Configuration: [Kr] 4d² 5s²
Complete Electron Configuration for Barium
Barium is a highly reactive alkaline earth metal with atomic number 56 and bears the symbol ‘Ba’. Since it is highly reactive, we cannot find this metal in its free state and always remains in combination with other metals.
Unabbreviated Electron Configuration: 1s2 2s²2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p⁶ 6s2
Abbreviated Electron Configuration: [Xe] 6s2
Complete Electron Configuration for Xenon
Xenon is a noble gas element that is available in very less amounts on the Earth’s crust. It holds the atomic number 54 and symbol ‘Xe’.
Unabbreviated Electron Configuration: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6
Abbreviated Electron Configuration: [Kr] 4d¹⁰ 5s² 5p⁶
Conclusion
The overall benefits of writing electron configurations for elements include:
- Helps in describing the valence of a particular element
- Useful for defining the chemical properties of elements that fall under same group in the periodic table.
- To find out elements that show similar chemical and physical properties.
- For understanding the complete picture of atomic spectra of elements in the periodic table.
- To differentiate the elements into different blocks and groups such as s-block, p-block, d-block and f-block elements.
The notation of writing electron configuration to an element has come into practice after the invention of Bohr Model of Atom theory by Niels Bohr.

