In this article, lets explore when mixing baking soda and vinegar does temperature change.

Let’s understand about baking soda a bit.
Baking soda often called by other names such as sodium bicarbonate or bicarbonate of soda.
Baking soda is an alkaline substance when mixed with acids change the pH levels and that is the reason it became a quickest remedy for illnesses like stomach upset, bad smell etc.
In Chemical composition – Baking soda is denoted as NaHCo3.
Hypothesis
When baking soda or sodium Bicarbonate (NaHCo3) reacts with water resulting in release of carbonic acid.
The reaction is exothermic that means it gets the heat out. Baking soda is just sodium bicarbonate.
It will dissolve in water making it slightly more basic since sodium bi carbonate is alkaline in nature. So, I believe the temperature of water decreases when the baking soda is added.
Supplies required for the Experiment

We require very few supplies that are available around our kitchen. Let us gather our simple ingredients to our experiment table. Find them below:
- Water
- Lemon Juice (Fresh Ones)
- Baking Soda
- Vinegar
- Glass Jars
- Stirring Rod (You can use spoon in your kitchen)
- Thermometer (to note down the temperature recordings)
Impact of Baking Soda and Vinegar on Temperature – Experiment Steps
Step-1: Take three glass Jar or Cup or Bowl on your experiment table. Make sure the glass bowl is clean and clear with no contaminants.
Step-2: Label the three glass bowls with the names ‘Fresh Water’, ‘Milk’, and ‘Lemon Juice’.

Step-3: Take three equal quantities of baking soda in three separate bowls.
Step-4: Now measure and take equal quantities of fresh water, fresh milk, and fresh lemon juice. You can use your measuring scoops for measuring. Make it simple. No need to bother much on this step.

Step-5: Now our experiment glasses are ready to experiment. Add the measured equal quantities of baking soda to all the three glasses separately but at the same time. Observe the reactions taking place without missing.
Step-6: You can see the bubbles formation inside the glasses as soon as you add the baking soda.

Kids are amazed to see such reactions with easy and simple experiments. Though it is a demonstration for showing kids how exothermic reactions work, I do not suggest kids to involve completely handling baking soda.

Step-7: Now let us add few drops or spoonful of vinegar to the experiment glasses after adding baking soda. Vinegar increases the chemical reaction and works as an activator.

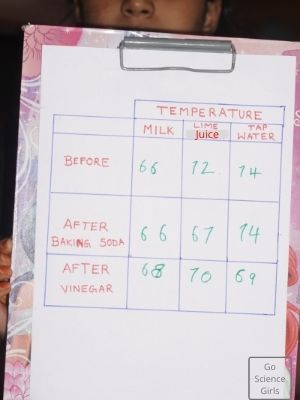
Observation and results
All the experiment liquids such as water, milk, lemon juice reacts with the baking soda added and gets heated up thus decreasing the temperature of the liquids.
This is observed by the formation of gas bubbles. Our hypothesis is proved right! Smiles!!

It’s time to know the science behind our Experiment Results
Acid. I’ve been done this a few times. It sizzle. The water that contains baking soda doesn’t bubble because it is an alkali, the chemical opposite of an acid.
When an alkali combines with an acid, it forms carbon dioxide. … When you add baking powder to water or milk, the alkali and the acid react with one another and produce carbon dioxide – the bubbles.


Baking soda means sodium bicarbonate which is alkaline in nature when reacts with water causes exothermic reaction.
An exothermic reaction is a chemical reaction that releases energy through light or heat. It is the opposite of an endothermic reaction. Also, some endothermic reactions happen during the reaction but the exothermic reactions dominate.
A carbonic acid is released when baking soda-water exothermic reaction happens which eventually releases carbon dioxide.
Carbon dioxide is something that releases heat into the surrounding atmosphere. That is the reason water gets heated when baking soda is added to it.
The rate of temperature rise depends on the amount of baking soda added. The same chemical reaction happens when baking soda is added to the milk and lemon juice. An acid-base reaction happens as soon as baking soda is added.

What happens when vinegar is added to baking soda?
The process of combining baking soda and vinegar forms a cloudy liquid and produces carbon dioxide.
The acid-based chemical reaction happens between baking soda and vinegar because baking soda is a base whereas vinegar is acid.
This acid-based chemical reaction is exothermic and releases heat energy through the release of carbon dioxide.
That’s the magic behind baking soda and vinegar reactions with water. Hope you all understood the simple science and now you are able to explain your kids on hands-on simple science experiments.

Take it further
Take different quantities of experiment liquids and try adding different quantities of baking soda and observe the results.
Try adding salt and sugar to the water before adding baking soda and observe the results and what happens before and after adding baking soda to the salt and sugar water.
Use different solids that dissolve in water and see what you get after using baking soda. Also, try mixing oil to the water before you add baking soda.
The colder vinegar is not a good option to produce more carbon dioxide.
The reaction between vinegar and baking soda (bicarbonate soda) is endothermic reaction.
Endothermic reaction is something that absorbs energy from the surroundings especially in the form of heat to produce products. Finally, we can consider cold vinegar is a disadvantage.
Baking soda also known as sodium bicarbonate is a household chemical mostly used for cooking and a lot many other purposes.
The melting point of baking soda is any temperature over 50 degrees Celsius. At 50 degrees Celsius, it starts to undergo thermal decomposition into sodium carbonate, water, and carbon dioxide when heated to above 50 degrees Celsius in the process.
The long term and over use of baking soda can increase may increase your potential risk to some of health issues such as hypernatremia (rise in sodium levels), worsening heart failure, hypokalaemia (potassium blood deficiency), worsening kidney disease, muscle weakness and cramps, hypochloremia (chloride blood deficiency), increased stomach acid production etc.
The full chemical reaction of baking soda and vinegar is as follows: Baking soda (sodium bicarbonate, NaHCO3) and vinegar (dilute acetic acid, CH3COOH) react to form three compounds such as Sodium acetate (CH3COONa), water (H2O), and carbon dioxide gas (CO2).
The carbon dioxide gas released during the process is used in chemical volcanoes and other projects. The products of reaction are relatively safe.
Cleaning with baking soda and vinegar is really helpful and useful, but only if you know when it works effectively and when it doesn’t! Combine 1/2 cup of dish soap with 1 2/3 cup of baking soda in a bowl. Also, add 1/2 cup of water followed by 3 tablespoons of vinegar to the bowl and continue mixing to combine the ingredients well and to get rid of any lumps.
Safety Guide
Protective and experimental gloves and clothing should be worn while performing experiment. Since baking soda is most-often seen in a powder-like state, make sure the fan and any air generators must be switched off while experimenting. Also, chemical safety goggles should be worn and be sure the area to wash eyes and skin is located near your work area. Because if this powder gets into a worker’s eyes, it can cause mild to moderate irritation. Keep your children handle baking soda with utmost care. Younger kids are suggested not to touch the powder without proper safety.
